Information
Version: B | 1.1 (2022-06-23)
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
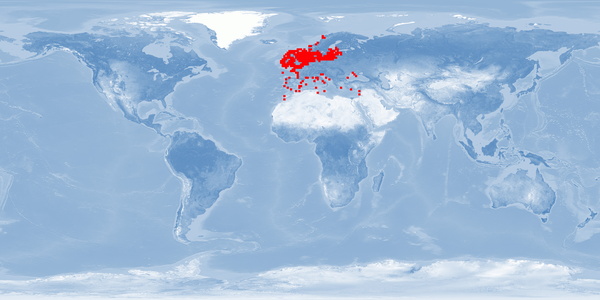
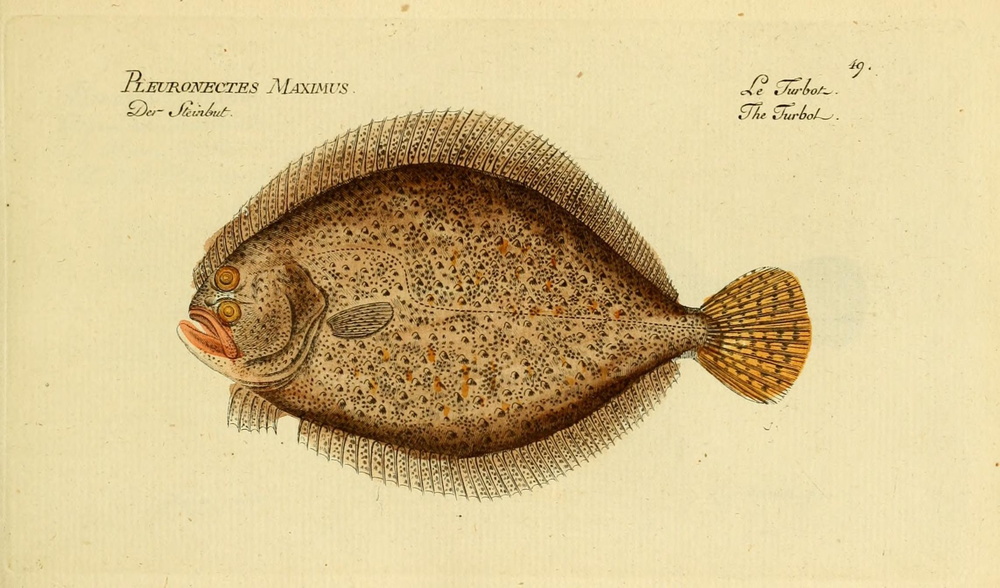
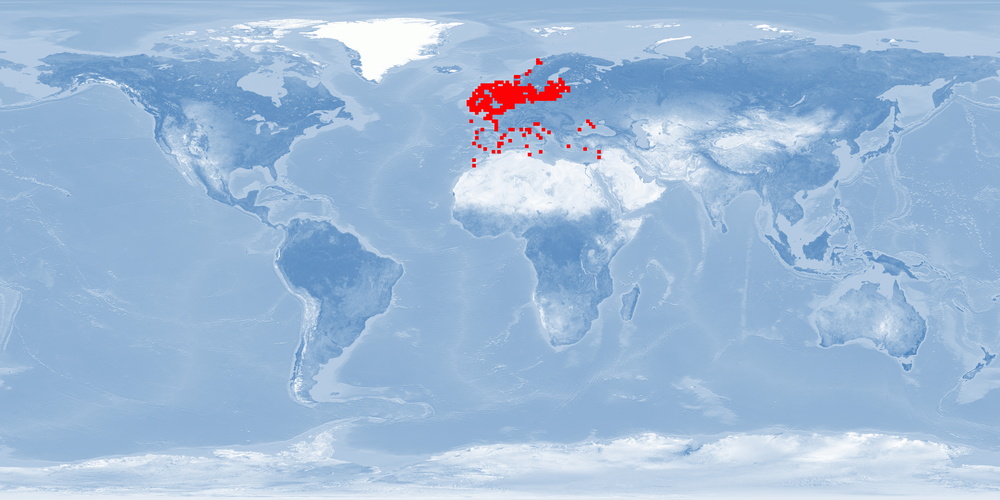
Scophthalmus maximus is a left-eyed flatfish of the family Scophtalmidae, a DEMERSAL predator, mainly in the area from the Black and Mediterranean Sea to the North Atlantic and the Baltic Sea. S. maximus is a relatively scarce “gourmet” fish and is highly prized in the market. S. maximus farming started in the 1970s in the United Kingdom and subsequently established in France, Spain, and Portugal. In the beginning, the production was restricted by a limited juvenile supply, but technological developments since 2007 have led to an expansion in production. Only few findings are available on both natural behaviour and physiological effects of farming practices. They clearly demonstrate the importance of sandy substrate and low stocking densities to improve fish welfare.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a high amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a low amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is unclear for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a low amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 3 37, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
WILD: carnivorous 5. FARM: for JUVENILES and ADULTS, fish meal and fish oil may be partly* replaced by non-forage fishery components 38 39 40 41. For LARVAE and SPAWNERS, no data found yet on replacement of fish meal and fish oil.
*partly = <51% – mostly = 51-99% – completely = 100%
Glossary
12L = 12 h light
20L = 20 h light
4D = 4 h dark
ADULTS = mature individuals, for details ➝ Findings 10.1 Ontogenetic development
DEMERSAL = living and feeding on or near the bottom of a body of water, mostly benthopelagic, some benthic
DOMESTICATION LEVEL 3 = entire life cycle closed in captivity with wild inputs 37
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
OCEANODROMOUS = living and migrating in the sea
PHOTOPERIOD = duration of daylight
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Aneer, Gunnar, and Lars Westin. 1990. Migration of turbot (Psetta maxima L.) in the northern Baltic proper. Fisheries Research 9: 307–315. https://doi.org/10.1016/0165-7836(90)90049-2.
3 Støttrup, J. G, C. R Sparrevohn, J Modin, and K Lehmann. 2002. The use of releases of reared fish to enhance natural populations: A case study on turbot Psetta maxima (Linné, 1758). Fisheries Research 59: 161–180. https://doi.org/10.1016/S0165-7836(01)00413-1.
4 Chen, J., Guang, C., Xu, H, Chen, Z., Xu, P., Yan, X., Wang, Y., and Liu, J. 2008. Marine fish cage culture in China. In FAO Fisheries Proceedings, 11:285–299. Guangzhou, China: FAO Fisheries and Aquaculture Department.
5 Daniels, H.V., and W.O. Watanabe. 2010. Practical Flatfish Culture and Stock Enhancement. Wiley-Blackwell.
6 Martinsson, Jesper, and Anders Nissling. 2011. Nursery area utilization by 0-group turbot (Psetta maxima) and flounder (Platichthys flesus) at Gotland, Baltic Sea. Boreal environment research 16: 60–70.
7 Güneş, E., and T. Şahin. 2012. Distribution and Abundance of Turbot (Psetta maxima) in the Southeastern Black Sea. Turkish Journal of Science & Technology 7: 19–30.
8 Panayotova, M.D., V.S. Raykov, and V.R. Todorova. 2012. Turbot (Psetta maxima L.) Abundance Indices and Stock Dynamics of Bulgarian Black Sea Coast During the Period 2006-2009. Acta Zoologica Bulgaria 64: 85–91.
9 Brown, Nick. 2002. Flatfish farming systems in the Atlantic region. Reviews in Fisheries Science 10: 403–419. https://doi.org/10.1080/20026491051712.
10 Van der Land, Marco A. 1991. Distribution of flatfish eggs in the 1989 egg surveys in the southeastern North Sea, and mortality of plaice and sole eggs. Netherlands Journal of Sea Research 27. Proceedings of the First International Symposium on Flatfish Ecology: 277–286. https://doi.org/10.1016/0077-7579(91)90030-5.
11 Basaran, Fatih, and Necati Samsun. 2004. Survival Rates of Black Sea Turbot (Psetta maxima maeotica, L. 1758) Broodstock Captured by Gill Nets from Different Depths and their Adaptation Culture Conditions. Aquaculture International 12: 321–331. https://doi.org/10.1023/B:AQUI.0000036183.39217.2a.
12 Florin, A. B. 2005. Flatfishes in the Baltic Sea - a review of biology and fishery with a focus on Swedish conditions - Purchase PDF. 14. Finfo Fiskeriverket.
13 Stefánsson, M Ö, R D FitzGerald, and T F Cross. 2002. Growth, feed utilization and growth heterogeneity in juvenile turbot Scophthalmus maximus (Rafinesque) under different photoperiod regimes. Aquaculture Research 33: 177–187. https://doi.org/10.1046/j.1365-2109.2002.00651.x.
14 Caputo, Vincenzo, Giacomo Candi, Sabrina Colella, and Enrico Arneri. 2001. Reproductive biology of turbot (Psetta maxima) and brill (Scophthalmus rhombus) (Teleostei, Pleuronectiformes) in the Adriatic Sea. Italian Journal of Zoology 68: 107–113. https://doi.org/10.1080/11250000109356393.
15 Aydin, I., and T. Sahin. 2011. Reproductive performance of turbot (Psetta maxima) in the southeastern Black Sea. Turk J Zool 35: 109–113.
16 Hara, S., M. Özongun, E. Günes, and B. Ceylan. 2002. Broodstock rearing ans spawning of Black Sea Turbot, Psetta maxima. Turkish Journal of Fisheries and Aquatic Science 2: 9–2.
17 Person Le-Ruyet, J. 2002. Turbot (Scophthalmus maximus) grow-out in Europe: practices, results, and prospects. Turkish Journal of Fisheries and Aquatic Science: 29–39.
18 Aksungur, Nilgün, Muharrem Aksungur, Bilal Akbulut, and İlyas Kutlu. 2007. Effects of Stocking Density on Growth Performance, Survival and Food Conversion Ratio of Turbot (Psetta maxima) in the Net Cages on the Southeastern Coast of the Black Sea. Turkish Journal of Fisheries and Aquatic Sciences 7: 147–152.
19 Irwin, S., J. O’Halloran, and R.D. FitzGerald. 1999. Stocking density, growth and growth variation in juvenile turbot, Scophthalmus maximus (Rafinesque). Aquaculture 178: 77–88.
20 Gonçalves, José Fernando Magalhães, Bruno Graziano da Silva Turini, and Rodrigo Otávio de Almeida Ozório. 2010. Performance of juvenile turbot (Scophthalmus maximus) fed varying dietary L-carnitine levels at different stocking densities. Scientia Agricola 67: 151–157. https://doi.org/10.1590/S0103-90162010000200004.
21 Jia, Rui, Bao-Liang Liu, Wen-Rong Feng, Cen Han, Bin Huang, and Ji-Lin Lei. 2016. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish & Shellfish Immunology 55: 131–139. https://doi.org/10.1016/j.fsi.2016.05.032.
22 Martınes-Tapia, C., and Fernandez-Pato, C.A. 1991. Influence of stock density on turbot (Scophthalmus maximus L.) growth. Mariculture Commitee CM1991/F:20. Spain: ICES.
23 Sunde, Leif M., Albert K. Imsland, Arild Folkvord, and Sigurd O. Stefansson. 1998. Effects of size grading on growth and survival of juvenile turbot at two temperatures. Aquaculture International 6: 19–32. https://doi.org/10.1023/A:1009265602388.
24 Sæther, B-S., and M. Jobling. 1999. The effects of ration level on feed intake and growth, and compensatory growth after restricted feeding, in turbot Scophthalmus maximus L. Aquaculture Research 30: 647–653. https://doi.org/10.1046/j.1365-2109.1999.00368.x.
25 Sparrevohn, C. R., and J. G. Støttrup. 2003. Bottom substrate preference in wild and reared turbot Psetta maxima L. Journal of Fish Biology 63: 257–257. https://doi.org/10.1111/j.1095-8649.2003.216bs.x.
26 Kristensen, Louise Dahl, Claus Reedtz Sparrevohn, Jens Tang Christensen, and Josianne Gatt Støttrup. 2014. Cryptic Behaviour of Juvenile Turbot Psetta maxima L. and European Flounder Platichthys flesus L. Open Journal of Marine Science 04: 185. https://doi.org/10.4236/ojms.2014.43018.
27 Arechavala-Lopez, Pablo. 2017. Personal communication.
28 Waring, Colin P., Michael G. Poxton, and Ronald M. Stagg. 1997. The physiological response of the turbot to multiple net confinements. Aquaculture International 5: 1–12. https://doi.org/10.1007/BF02764783.
29 NOT FOUND
30 Gaumet, F., G. Boeuf, J. -P. Truchot, and G. Nonnotte. 1994. Effects of environmental water salinity on blood acid-base status in juvenile turbot (Scophthalmus maximus L.). Comparative Biochemistry and Physiology Part A: Physiology 109: 985–994. https://doi.org/10.1016/0300-9629(94)90247-X.
31 Staurnes, Magne. 1994. Effects of acute temperature decreases on turbot fry and juveniles. Aquaculture International 2: 104–113. https://doi.org/10.1007/BF00128804.
32 Üstündağ, C., Y. Çiftci, and F. Sakamoto. 2002. Rearing of Larvae and Juveniles of Black Sea Turbot, Psetta maxima, in Turkey. Turkish Journal of Fisheries and Aquatic Science 2: 13–17.
33 Knowles, Toby G, Steve N Brown, Paul D Warriss, Jeff Lines, Ambrose Tinarwo, and Marta Sendon. 2008. Effect of electrical stunning at slaughter on the quality of farmed turbot (Psetta maxima). Aquaculture Research 39: 1731–1738. https://doi.org/10.1111/j.1365-2109.2008.02049.x.
34 Roth, B., A. K. Imsland, and A. Foss. 2009. Live chilling of turbot and subsequent effect on behaviour, muscle stiffness, muscle quality, blood gases and chemistry. Universities Federation for Animal Welfare 1: 33–41.
35 Morzel, Martine, Delphine Sohier, and Hans Van de Vis. 2003. Evaluation of slaughtering methods for turbot with respect to animal welfare and flesh quality. Journal of the Science of Food and Agriculture 83: 19–28. https://doi.org/10.1002/jsfa.1253.
36 Lambooij, Bert, Hanne Digre, Ulf Erikson, Henny Reimert, Dirk Burggraaf, and Hans van de Vis. 2013. Evaluation of Electrical Stunning of Atlantic Cod (Gadus morhua) and Turbot (Psetta maxima) in Seawater. Journal of Aquatic Food Product Technology 22: 371–379. https://doi.org/10.1080/10498850.2011.654047.
37 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
38 Burel, Christine, Thierry Boujard, Sadasivam J Kaushik, Gilles Boeuf, Serge Van Der Geyten, Koen A Mol, Eduard R Kühn, Alain Quinsac, Michel Krouti, and Daniel Ribaillier. 2000. Potential of plant-protein sources as fish meal substitutes in diets for turbot (Psetta maxima): growth, nutrient utilisation and thyroid status. Aquaculture 188: 363–382. https://doi.org/10.1016/S0044-8486(00)00342-2.
39 Regost, C, J Arzel, J Robin, G Rosenlund, and S. J Kaushik. 2003. Total replacement of fish oil by soybean or linseed oil with a return to fish oil in turbot (Psetta maxima): 1. Growth performance, flesh fatty acid profile, and lipid metabolism. Aquaculture 217: 465–482. https://doi.org/10.1016/S0044-8486(02)00259-4.
40 Fournier, V, C Huelvan, and E Desbruyeres. 2004. Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psetta maxima). Aquaculture 236: 451–465. https://doi.org/10.1016/j.aquaculture.2004.01.035.
41 Kroeckel, S., A.-G.E. Harjes, I. Roth, H. Katz, S. Wuertz, S. Susenbeth, and C. Schulz. 2012. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute — Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364–365: 345–352.