Information
Version: C | 1.0 (2023-12-31)
- profile update resulting in major editorial and content changes (changing the scoring in criteria 1-3, 5-10)
- transfer to consistent age class and label structure resulting in changed appearance
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
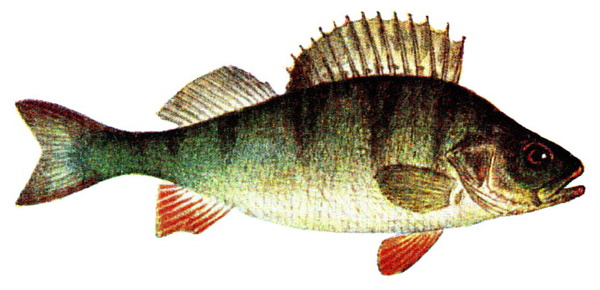
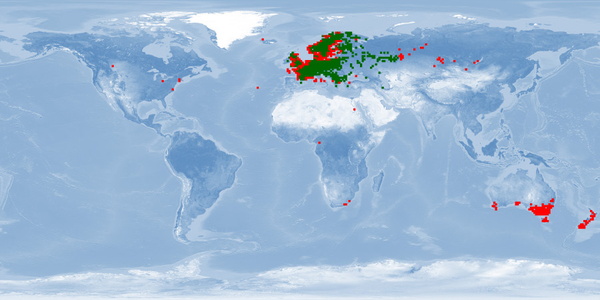
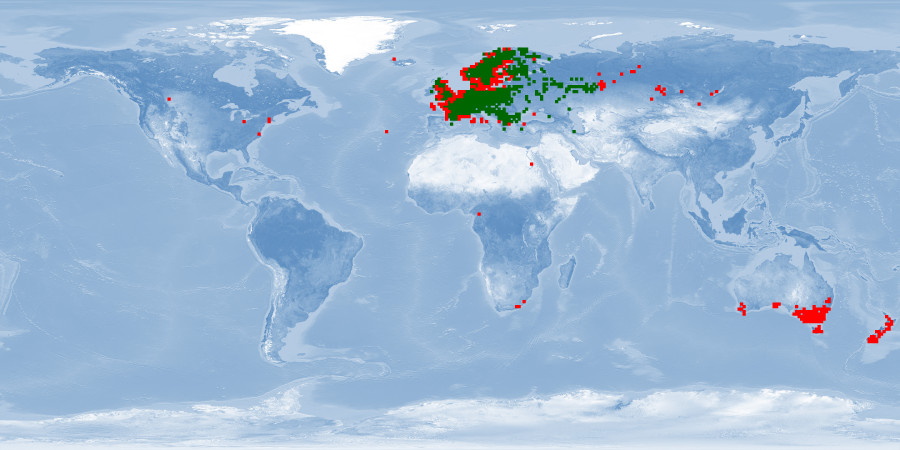
Perca fluviatilis is a percid species that inhabits Eurasian inland and coastal waters and has been introduced in inland waters worldwide. The production of P. fluviatilis has emerged over the past decades while important biological processes of the species are not known yet. P. fluviatilis is a strong predator in the wild, and as such, an aggressive and cannibalistic fish in captivity – a constraint that is not properly prevented in farms yet. In fact, prey FISHES (e.g., roach, Rutilus rutilus, topmouth gudgeon, Pseudorasbora parva or other small cyprinids species) are added in monoculture systems to satiate the predatory nature of P. fluviatilis. It is also susceptible to stress and malformations in captivity. Tanks or raceways will most likely not fulfil space needs in intensive conditions. The biggest knowledge gap is on humane slaughter practices for this species. Further research is needed on both natural behaviour and physiological effects of farming practices in order to provide recommendations for improving fish welfare.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal farming conditions, as tanks, RAS, and some ponds do not cover the whole range in the wild. It is medium for high-standard farming conditions, as other ponds at least overlap with the range in the wild, although we cannot be sure in most age classes. Our conclusion is based on a medium amount of evidence, as further wild information is missing in LARVAE, FRY, and JUVENILES.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions, as cages, ponds, and tanks do not cover the whole range in the wild. It is medium for high-standard farming conditions, as ponds overlap with the range in the wild. Our conclusion is based on a high amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions, as the ANADROMOUS strain undertakes more or less extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. It is high for high-standard farming conditions given the resident strain. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions, as the species is manipulated (hormonal manipulation, stripping) and may be taken from the wild. It is high for high-standard farming conditions, as natural breeding with farm-reared IND is possible and verified for the farming context. Our conclusion is based on a medium amount of evidence, as further research is needed on reproduction behaviour in the wild.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as densities in tanks and some ponds go beyond the minimum density in the wild. It is high for high-standard farming conditions, as densities in other ponds potentially cover the range in the wild. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions, as the species is aggressive – even cannibalistic – in almost all age classes. It is medium for high-standard farming conditions, as ways to reduce (but not avoid) cannibalism (size homogeneity, reducing densities) are verified for the farming context. Our conclusion is based on a high amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as almost all age classes of the species use substrate, but RAS and some ponds are devoid of it. It is high for high-standard farming conditions given a) hatching substrate for eggs, b) earthen ponds for JUVENILES and ADULTS which are not lined, and given b) natural reproduction with spawning substrate in ponds for SPAWNERS. Our conclusion is based on a high amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions, as the species is stressed (confinement, handling, husbandry). It is medium for high-standard farming conditions, as improvements are easily imaginable, but need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions, as malformation rates may exceed 10%. It is medium for high-standard farming conditions, as some malformations result from conditions that may be changed (rearing environment, feed). Our conclusion is based on a medium amount of evidence, as improvement of the situation by adjusting conditions needs more proof.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is unclear for minimal and high-standard farming conditions, although percussive stunning followed by bleeding seems promising, but needs to be verified for the farming context. Our conclusion is based on a low amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 101 102, level 5 being fully domesticated. Cultured since 1950 103.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: carnivorous and piscivorous, mainly zooplankton as JUVENILES, increasing proportion of fish with increasing age 49 26 32 82.
- FARM: limited application of fish oil replacements due to alterations in liver structure 104. JUVENILES: fish meal may be not 105 to partly* replaced by non-forage fishery components 106.
- LAB: FINGERLINGS: fish meal may be not replaced by sustainable sources 107. JUVENILES: fish meal may be partly* replaced by non-forage fishery components 108 109 110.
* partly = <51% – mostly = 51-99% – completely = 100%
Glossary
ANADROMOUS = migrating from the sea into fresh water to spawn
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 101
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger
FISHES = Using "fishes" instead of "fish" for more than one individual - whether of the same species or not - is inspired by Jonathan Balcombe who proposed this usage in his book "What a fish knows". By referring to a group as "fishes", we acknowledge the individuals with their personalities and needs instead of an anonymous mass of "fish".
FRY = larvae from external feeding on
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
RAS = Recirculating Aquaculture System - almost completely closed system using filters to clean and recirculate water with the aim of reducing water input and with the advantage of enabling close control of environmental parameters to maintain high water quality
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson) 76
WILD = setting in the wild
Bibliography
2 Kestemont, P., C. Mélard, J. A. Held, and K. Dabrowski. 2015. Culture Methods of Eurasian Perch and Yellow Perch Early Life Stages. In Biology and Culture of Percid Fishes, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 265–293. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-7227-3_9.
3 Kestemont, Patrick, Carole Rougeot, Jiri Musil, and Damien Toner. 2008. Larval and Juvenile Production. In Farming of Eurasian Perch, ed. Damien Toner and Carole Rougeot, 1-Juvenile Production:30–41. Aquaculture Explained 24. Dublin: Aquaculture Development Division, Bord Iascaigh.
4 Lahnsteiner, Franz, and Manfred Kletzl. 2018. A method for Rearing Perch, Perca fluviatilis, Larvae Using Paramecium caudatum, Followed by Wild Zooplankton and Formulated Dry Feed in Combination With Adequate Tank Systems. Journal of Agricultural Science 10: 26. https://doi.org/10.5539/jas.v10n8p26.
5 Jacquemond, François. 2004. Separated breeding of perch fingerlings (Perca fluviatilis L.) with and without initial inflated swim bladder: comparison of swim bladder development, skeleton conformation and growth performances. Aquaculture 239: 261–273. https://doi.org/10.1016/j.aquaculture.2004.06.019.
6 Jankowska, Barbara, Zdzisław Zakęś, Tomasz Żmijewski, Szczepkowski Mirosław, and Agata Cejko. 2007. Slaughter yield, proximate composition, and flesh colour of cultivated and wild perch (Perca fluviatilis L.). Czech Journal of Animal Science 52: 260–267. https://doi.org/10.17221/2279-CJAS.
7 Policar, Tomas, Damien Toner, S.M.H. Alavi, and Ottomar Linhart. 2008. Reproduction and Spawning. In Farming of Eurasian Perch, ed. Damien Toner and Carole Rougeot, 1-Juvenile Production:22–29. Aquaculture Explained 24. Dublin: Aquaculture Development Division, Bord Iascaigh.
8 Policar, Tomáš, Azin Mohagheghi Samarin, and Charles Mélard. 2015. Culture Methods of Eurasian Perch During Ongrowing. In Biology and Culture of Percid Fishes, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 417–435. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-7227-3_16.
9 Eklöv, P. 1997. Effects of habitat complexity and prey abundance on the spatial and temporal distributions of perch (Perca fluviatilis) and pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 54: 1520–1531. https://doi.org/10.1139/cjfas-54-7-1520.
10 Linløkken, Arne, Eva Bergman, Larry Greenberg, and Per Arne Holt Seeland. 2008. Environmental correlates of population variables of perch (Perca fluviatilis) in boreal lakes. Environmental Biology of Fishes 82: 401–408. https://doi.org/10.1007/s10641-007-9301-y.
11 Francová, Kateřina, and Markéta Ondračková. 2014. Effect of habitat conditions on parasite infection in 0+ juvenile perch (Perca fluviatilis L.) in two Czech reservoirs. Hydrobiologia 721. https://doi.org/10.1007/s10750-013-1644-0.
12 Bochert, Ralf. 2022. Comparative performance, biochemical composition, and fatty acid analysis of Eurasian perch (Perca fluviatilis) during grow-out in RAS fed different commercial diets. Journal of Applied Aquaculture 34: 208–222. https://doi.org/10.1080/10454438.2020.1828217.
13 Monk, Christopher T., and Robert Arlinghaus. 2017. Eurasian perch, Perca fluviatilis, spatial behaviour determines vulnerability independent of angler skill in a whole-lake reality mining experiment. Canadian Journal of Fisheries and Aquatic Sciences 75: 417–428. https://doi.org/10.1139/cjfas-2017-0029.
14 Westrelin, Samuel, Romain Roy, Laurence Tissot-Rey, Laurent Bergès, and Christine Argillier. 2018. Habitat use and preference of adult perch (Perca fluviatilis L.) in a deep reservoir: variations with seasons, water levels and individuals. Hydrobiologia 809: 121–139. https://doi.org/10.1007/s10750-017-3454-2.
15 Fontaine, Pascal, Patrick Kestemont, and Charles Mélard. 2008. Broodstock Management. In Farming of Eurasian Perch, ed. Damien Toner and Carole Rougeot, 1-Juvenile Production:16–21. Aquaculture Explained 24. Dublin: Aquaculture Development Division, Bord Iascaigh.
16 Křišťan, Jiří, Vlastimil Stejskal, and Tomáš Policar. 2012. Comparison of Reproduction Characteristics and Broodstock Mortality in Farmed and Wild Eurasian Perch (Perca fluviatilis L.) Females During Spawning Season Under Controlled Conditions. Turkish Journal of Fisheries and Aquatic Sciences 12: 191–197.
17 Steenfeldt, Svend, Pascal Fontaine, Julia Lynne Overton, Tomáš Policar, Damien Toner, Bahram Falahatkar, Ákos Horváth, Ines Ben Khemis, Neila Hamza, and Mohammed Mhetli. 2015. Current Status of Eurasian Percid Fishes Aquaculture. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 817–841. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-7227-3_32.
18 Skovrind, Mikkel, Emil A. F. Christensen, Henrik Carl, Lene Jacobsen, and Peter R. Møller. 2013. Marine spawning sites of perch Perca fluviatilis revealed by oviduct-inserted acoustic transmitters. Aquatic Biology 19: 201–206. https://doi.org/10.3354/ab00529.
19 Thorpe, John. 1977. Synopsis of biological data on the perch: Perca fluviatilis Linnaeus, 1758 and Perca flavescens Mitchill, 1814. FAO Fisheries Synopsis 113. Rome, Italy: Food and Agriculture Organization of the United Nations.
20 Čech, Martin, Jiří Peterka, Milan Říha, Vladislav Draštík, Michal Kratochvíl, and Jan Kubečka. 2010. Deep spawning of perch (Perca fluviatilis, L.) in the newly created Chabařovice Lake, Czech Republic. Hydrobiologia 649: 375–378. https://doi.org/10.1007/s10750-010-0251-6.
21 Viljanen, Markku, and Ismo J. Holopainen. 1982. Population density of perch (Perca fluviatilis L.) at egg, larval and adult stages in the dys-oligotrophic Lake Suomunjärvi, Finland. Annales Zoologici Fennici 19: 39–46.
22 Treasurer, James W. 1983. Estimates of egg and viable embryo production in a lacustrine perch, Perca fluviatilis. Environmental Biology of Fishes 8: 3–16. https://doi.org/10.1007/BF00004941.
23 Gillet, C., and J. P. Dubois. 1995. A survey of the spawning of perch (Perca fluviatilis), pike (Esox lucius), and roach (Rutilus rutilus), using artificial spawning substrates in lakes. In Space Partition within Aquatic Ecosystems, ed. Gérard Balvay, 409–415. Developments in Hydrobiology 104. Springer Netherlands. https://doi.org/10.1007/978-94-011-0293-3_39.
24 Dubois, Jean-Paul, Christian Gillet, Stéphane Bonnet, and Yvette Chevalier-Weber. 1996. Correlation between the size of mature female perch (Perca fluviatilis L.) and the width of their egg strands in Lake Geneva. Annales Zoologici Fennici 33: 417–420.
25 Probst, W. N., S. Stoll, H. Hofmann, P. Fischer, and R. Eckmann. 2009. Spawning site selection by Eurasian perch (Perca fluviatilis L.) in relation to temperature and wave exposure. Ecology of Freshwater Fish 18: 1–7. https://doi.org/10.1111/j.1600-0633.2008.00327.x.
26 Persson, Lennart, Pär Byström, and Eva Wahlström. 2000. Cannibalism and Competition in Eurasian Perch: Population Dynamics of an Ontogenetic Omnivore. Ecology 81: 1058–1071. https://doi.org/10.1890/0012-9658(2000)081[1058:CACIEP]2.0.CO;2.
27 Petrtýl, Miloslav, Lukáš Kalous, Jaroslava Frouzová, and Martin Čech. 2015. Effects of habitat type on short- and long-term growth parameters of the European perch (Perca fluviatilis L.): Size distribution and RNA/DNA ratio of European perch fry. International Review of Hydrobiology 100: 13–20. https://doi.org/10.1002/iroh.201301675.
28 Čech, M., J. Kubečka, J. Frouzová, V. Draštík, M. Kratochvíl, J. Matěna, and J. Hejzlar. 2007. Distribution of the bathypelagic perch fry layer along the longitudinal profile of two large canyon-shaped reservoirs. Journal of Fish Biology 70: 141–154. https://doi.org/10.1111/j.1095-8649.2006.01282.x.
29 Craig, John F. 1977. Seasonal changes in the day and night activity of adult perch, Perca fluviatilis L. Journal of Fish Biology 11: 161–166.
30 Jellyman, D. J. 1980. Age, growth, and reproduction of perch, Perca fluviatilis L., in Lake Pounui. New Zealand Journal of Marine and Freshwater Research 14: 391–400. https://doi.org/10.1080/00288330.1980.9515881.
31 Egloff, Mathias. 1996. Failure of swim bladder inflation of perch, Perca fluviatilis L. found in natural populations. Aquatic Sciences 58: 15–23.
32 Ceccuzzi, Pietro, Genciana Terova, Fabio Brambilla, Micaela Antonini, and Marco Saroglia. 2011. Growth, diet, and reproduction of Eurasian perch Perca fluviatilis L. in Lake Varese, northwestern Italy. Fisheries Science 77: 533–545. https://doi.org/10.1007/s12562-011-0353-8.
33 Ferter, Keno, and Victor Benno Meyer-Rochow. 2010. Turning Night into Day: Effects of Stress on the Self-Feeding Behaviour of the Eurasian Perch Perca fluviatilis. Zoological Studies 49: 176–181.
34 Allen, K. R. 1935. The Food and Migration of the Perch (Perca fluviatilis) in Windermere. Journal of Animal Ecology 4: 264–273. https://doi.org/10.2307/1016.
35 Čech, M., J. Peterka, M. Říha, L. Vejřík, T. Jůza, M. Kratochvíl, V. Draštík, M. Muška, P. Znachor, and J. Kubečka. 2012. Extremely shallow spawning of perch (Perca fluviatilis L.): the roles of sheltered bays, dense semi-terrestrial vegetation and low visibility in deeper water. Knowledge and Management of Aquatic Ecosystems 406: 12. https://doi.org/10.1051/kmae/2012026.
36 Cabrera-Álvarez, María. 2023. Conclusion.
37 Froese, R., and D. Pauly. 2017. Perca fluviatilis summary page. World Wide Web electronic publication. www.fishbase.org.
38 Kratochvil, M., J. Peterka, J. Kubecka, J. Matena, M. Vasek, I. Vanickova, M. Cech, and Jaromir Seda. 2008. Diet of larvae and juvenile perch, Perca fluviatilis performing diel vertical migrations in a deep reservoir. Folia Zoologica 57: 313–323.
39 Craig, J. F. 1974. Population dynamics of perch, Perca fluviatilis L. in Slapton Ley, Devon. I. Trapping behaviour, reproduction, migration, population estimates, mortality and food. Freshwater Biology 4: 417–431. https://doi.org/10.1111/j.1365-2427.1974.tb00106.x.
40 Le Cren, E. D. 1958. Observations on the Growth of Perch (Perca fluviatilis L.) Over Twenty-Two Years with Special Reference to the Effects of Temperature and Changes in Population Density. Journal of Animal Ecology 27: 287–334. https://doi.org/10.2307/2242.
41 Komolka, Katrin, Ralf Bochert, George P. Franz, Yagmur Kaya, Ralf Pfuhl, and Bianka Grunow. 2020. Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture. Foods 9: 388. https://doi.org/10.3390/foods9040388.
42 Järv, Leili. 2000. Migration of the perch (Perca fluviatilis L.) in the coastal waters of Western Estonia. In Proceedings of the Estonian Academy of Sciences, Biology and Ecology. Estonian Academy Publishers.
43 Järv, L. 2000. Perch Perca fluviatilis L. in Estonian coastal waters. Science Biology Ecology 49. Proc. Estonian Acad.: 270–276.
44 Gerlach, Gabriele, Uwe Schardt, Reiner Eckmann, and Axel Meyer. 2001. Kin-structured subpopulations in Eurasian perch (Perca fluviatilis L.). Heredity 86: 213–221.
45 Nesbø, C. L., C. Magnhagen, and K. S. Jakobsen. 1998. Genetic Differentiation Among Stationary and Anadromous Perch (Perca Fluviatilis) in the Baltic Sea. Hereditas 129: 241–249. https://doi.org/10.1111/j.1601-5223.1998.00241.x.
46 Snickars, Martin, Göran Sundblad, Alfred Sandström, Lars Ljunggren, Ulf Bergström, Gustav Johansson, and Johanna Mattila. 2010. Habitat selectivity of substrate-spawning fish: Modeling requirements for the Eurasian perch Perca fluviatilis. Marine Ecology Progress Series 398: 235–243. https://doi.org/10.3354/meps08313.
47 Tibblin, Petter, Per Koch-Schmidt, Per Larsson, and Patrik Stenroth. 2012. Effects of salinity on growth and mortality of migratory and resident forms of Eurasian perch in the Baltic Sea. Ecology of Freshwater Fish 21: 200–206. https://doi.org/10.1111/j.1600-0633.2011.00537.x.
48 Treasurer, J. W. 1981. Some aspects of the reproductive biology of perch Perca fluviatilis L. Fecundity, maturation and spawning behaviour. Journal of Fish Biology 18: 729–740. https://doi.org/10.1111/j.1095-8649.1981.tb03814.x.
49 Rask, Martti. 1983. Differences in growth of perch (Perca fluviatilis L.) in two small forest lakes. Hydrobiologia 101: 139–143. https://doi.org/10.1007/BF00008666.
50 Saemi Komsari, M., A. Bani, H. Khara, and H. Reza Esmaeili. 2014. Reproductive strategy of the European perch, Perca fluviatilis Linnaeus, 1758 (Osteichthyes: Percidae) in the Anzali wetland, southwest Caspian Sea. Journal of Applied Ichthyology 30: 307–313. https://doi.org/10.1111/jai.12335.
51 Voskoboinikova, O. S., and I. G. Grechanov. 2002. Development of the Skeleton during the Ontogenesis of the River Perch Perca fluviatilis. Journal of Ichthyology 42: 322–333.
52 Čech, Martin, Lukáš Vejřík, Jiří Peterka, Milan Říha, Milan Muška, Tomáš Jůza, Vladislav Draštík, Michal Kratochvíl, and Jan Kubečka. 2012. The use of artificial spawning substrates in order to understand the factors influencing the spawning site selection, depth of egg strands deposition and hatching time of perch (Perca fluviatilis L.). J. Limnol. 71: 18. https://doi.org/10.4081/jlimnol.2012.e18.
53 Hokanson, Kenneth E. F. 1977. Temperature Requirements of Some Percids and Adaptations to the Seasonal Temperature Cycle. Journal of the Fisheries Research Board of Canada 34: 1524–1550. https://doi.org/10.1139/f77-217.
54 Thorpe, J. E. 1977. Morphology, Physiology, Behavior, and Ecology of Perca fluviatilis L. and P. flavescens Mitchill. Journal of the Fisheries Research Board of Canada 34: 1504–1514. https://doi.org/10.1139/f77-215.
55 Craig, John F. 2000. Percid Fishes: Systematics, Ecology and Exploitation. John Wiley & Sons.
56 CABI. 2021. Perca fluviatilis (perch). CABI Compendium CABI Compendium: 70037. https://doi.org/10.1079/cabicompendium.70037.
57 Kucharczyk, D, R Kujawa, A Mamcarz, A Skrzypczak, and E Wyszomirska. 1998. Induced spawning in perch, Perca fluviatilis L., using FSH + LH with pimozide or metoclopramide. Aquaculture Research 29: 131–136. https://doi.org/10.1046/j.1365-2109.1998.00949.x.
58 Kouril, J., and J. Hamackova. 2000. The semiartificial and artificial hormonally induced propagation of European perch (Perca fluviatilis). In Proc. Aqua, 2000. Responsible Aquaculture in the New Millennium, ed. R. Floss and L. Creswell, 28:345. Spec. Publ. Oostende, Belgium.
59 Żarski, D., A. Horváth, J. A. Held, and D. Kucharczyk. 2015. Artificial Reproduction of Percid Fishes. In Biology and Culture of Percid Fishes - Principles and Practices, ed. P. Kestemont, K. Dabrowski, and Robert C. Summerfelt. Dordrecht: Springer Netherlands.
60 Rónyai, András, and Svetlana Anatolyevna Lengyel. 2010. Effects of hormonal treatments on induced tank spawning of Eurasian perch (Perca fluviatilis L.). Aquaculture Research 41: e345–e347. https://doi.org/10.1111/j.1365-2109.2009.02465.x.
61 Żarski, Daniel, Zoltán Bokor, László Kotrik, Béla Urbanyi, Akos Horváth, Katarzyna Targońska, Sławomir Krejszeff, Katarzyna Palińska, and Dariusz Kucharczyk. 2011. A new classification of a preovulatory oocyte maturation stage suitable for the synchronization of ovulation in controlled reproduction of Eurasian perch, Perca fluviatilis L. Reproductive Biology 11: 194–209. https://doi.org/10.1016/S1642-431X(12)60066-7.
62 Rougeot, Carole. 2015. Sex and Ploidy Manipulation in Percid Fishes. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 625–635. Dordrecht: Springer Netherlands.
63 Behrmann-Godel, Jasminca, Gabriele Gerlach, and Reiner Eckmann. 2006. Kin and Population Recognition in Sympatric Lake Constance Perch (Perca fluviatilis L.): Can Assortative Shoaling Drive Population Divergence? Behavioral Ecology and Sociobiology 59: 461–468.
64 Magnhagen, Carin. 2015. Behaviour of Percid Fishes in the Wild and Its Relevance for Culture. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 399–417. Dordrecht: Springer Netherlands.
65 Guillard, Jean, Patrice Brehmer, Michel Colon, and Yvon Guennégan. 2006. Three dimensional characteristics of young–of–year pelagic fish schools in lake. Aquatic Living Resources 19: 115–122. https://doi.org/10.1051/alr:2006011.
66 Čech, M., M. Kratochvíl, J. Kubečka, V. Draštík, and J. Matěna. 2005. Diel vertical migrations of bathypelagic perch fry. Journal of Fish Biology 66: 685–702. https://doi.org/10.1111/j.0022-1112.2005.00630.x.
67 Magnhagen, Carin, and Nils Bunnefeld. 2009. Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proceedings of the Royal Society of London B: Biological Sciences 276: 3369–3375. https://doi.org/10.1098/rspb.2009.0851.
68 Probst, Wolfgang Nikolaus, Gregor Thomas, and Reiner Eckmann. 2009. Hydroacoustic observations of surface shoaling behaviour of young-of-the-year perch Perca fluviatilis (Linnaeus, 1758) with a towed upward-facing transducer. Fisheries Research 96: 133–138. https://doi.org/10.1016/j.fishres.2008.10.009.
69 Overton, JL, and H Paulsen. 2005. Ongrowing of Perch (Perca fluviatilis) Juveniles (Videreopdræt af aborreyngel). No. 151-05. Danmarks Fiskeriundersøgelser, Afdeling for Havøkologi og Akvakultur, Bornholms Lakseklækkeri.
70 Janssens, Thomas. 2017. Personal communication.
71 Strand, Å., A. Alanärä, and C. Magnhagen. 2007. Effect of group size on feed intake, growth and feed efficiency of juvenile perch. Journal of Fish Biology 71: 615–619. https://doi.org/10.1111/j.1095-8649.2007.01497.x.
72 Schleuter, Diana, Susanne Haertel-Borer, Philipp Fischer, and Reiner Eckmann. 2007. Respiration Rates of Eurasian Perch Perca fluviatilis and Ruffe: Lower Energy Costs in Groups. Transactions of the American Fisheries Society 136: 43–55. https://doi.org/10.1577/T06-123.1.
73 Bruylants, B., A. Vandelannoote, and R. Verheyen. 1986. The movement pattern and density distribution of perch, Perca fluviatilis L., in a channelized lowland river. Aquaculture Research 17: 49–57. https://doi.org/10.1111/j.1365-2109.1986.tb00084.x.
74 Le Cren, E. D. 1992. Exceptionally big individual perch (Perca fluviatilis L.) and their growth. Journal of Fish Biology 40: 599–625. https://doi.org/10.1111/j.1095-8649.1992.tb02609.x.
75 Hematyar, Nima, Jan Mraz, Vlastimil Stejskal, Sabine Sampels, Zuzana Linhartová, Marketa Prokesova, Frantisek Vacha, et al. 2021. Comparison of Quality Changes in Eurasian Perch (Perca fluviatilis L.) Fillets Originated from Two Different Rearing Systems during Frozen and Refrigerated Storage. Foods 10: 1405. https://doi.org/10.3390/foods10061405.
76 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
77 Baras, Etienne, Patrick Kestemont, and Charles Mélard. 2003. Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 219: 241–255. https://doi.org/10.1016/S0044-8486(02)00349-6.
78 Król, Jaroslaw, Nicolas Dauchot, Syaghalirwa N M Mandiki, Pierre van Cutsem, and Patrick Kestemont. 2015. Cannibalism in cultured eurasian perch, Perca fluviatilis (Actinopterygii: Perciformes: Percidae)-Implication of maternal influence, kinship, and sex ratio of progenies. Acta Ichthyologica et Piscatoria 45: 65–73. https://doi.org/10.3750/AIP2015.45.1.07.
79 Westerberg, Magdalena, Fia Staffan, and Carin Magnhagen. 2004. Influence of predation risk on individual competitive ability and growth in Eurasian perch, Perca fluviatilis. Animal Behaviour 67: 273–279. https://doi.org/10.1016/j.anbehav.2003.06.003.
80 Magnhagen, C., and E. Heibo. 2004. Growth in length and in body depth in young-of-the-year perch with different predation risk. Journal of Fish Biology 64: 612–624. https://doi.org/10.1111/j.1095-8649.2004.00325.x.
81 Magnhagen, Carin, and Jost Borcherding. 2008. Risk-taking behaviour in foraging perch: does predation pressure influence age-specific boldness? Animal Behaviour 75: 509–517. https://doi.org/10.1016/j.anbehav.2007.06.007.
82 Heermann, Lisa, Werner Scharf, Gerard van der Velde, and Jost Borcherding. 2014. Does the use of alternative food resources induce cannibalism in a size-structured fish population? Ecology of Freshwater Fish 23: 129–140. https://doi.org/10.1111/eff.12060.
83 Mikheev, Victor N., Anna F. Pasternak, Gerhard Tischler, and Josef Wanzenböck. 2005. Contestable shelters provoke aggression among 0+ perch, Perca fluviatilis. Environmental Biology of Fishes 73: 227–231. https://doi.org/10.1007/s10641-005-0558-8.
84 Goldenberg, Silvan U., Jost Borcherding, and Martina Heynen. 2014. Balancing the response to predation—the effects of shoal size, predation risk and habituation on behaviour of juvenile perch. Behavioral Ecology and Sociobiology 68: 989–998. https://doi.org/10.1007/s00265-014-1711-1.
85 Staffan, F., C. Magnhagen, and A. Alanärä. 2002. Variation in food intake within groups of juvenile perch. Journal of Fish Biology 60: 771–774. https://doi.org/10.1111/j.1095-8649.2002.tb01702.x.
86 Treasurer, J. W., and F. G. T. Holliday. 1981. Some aspects of the reproductive biology of perch Perca fluviatilis L. A histological description of the reproductive cycle. Journal of Fish Biology 18: 359–376. https://doi.org/10.1111/j.1095-8649.1981.tb03778.x.
87 Jentoft, Sissel, Sigurd ØXnevad, Are H. Aastveit, and ØIvind Andersen. 2006. Effects of Tank Wall Color and Up-welling Water Flow on Growth and Survival of Eurasian Perch Larvae (Perca fluviatilis). Journal of the World Aquaculture Society 37: 313–317. https://doi.org/10.1111/j.1749-7345.2006.00042.x.
88 Borcherding, Jost. 2006. Prey or predator: 0+ perch (Perca fluviatilis) in the trade-off between food and shelter. Environmental Biology of Fishes 77: 87–96. https://doi.org/10.1007/s10641-006-9057-9.
89 Strand, Å., A. Alanärä, F. Staffan, and C. Magnhagen. 2007. Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis L. Aquaculture 272: 312–318. https://doi.org/10.1016/j.aquaculture.2007.08.052.
90 Douxfils, J., S. N. M. Mandiki, G. Marotte, N. Wang, F. Silvestre, S. Milla, E. Henrotte, et al. 2011. Does domestication process affect stress response in juvenile Eurasian perch Perca fluviatilis? Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 159: 92–99. https://doi.org/10.1016/j.cbpa.2011.01.021.
91 Haux, Carl, Maj-Lis Sjöbeck, and Åke Larsson. 1985. Physiological stress responses in a wild fish population of perch (Perca fluviatilis) after capture and during subsequent recovery. Marine Environmental Research 15: 77–95. https://doi.org/10.1016/0141-1136(85)90131-X.
92 Acerete, L, J. C Balasch, E Espinosa, A Josa, and L Tort. 2004. Physiological responses in Eurasian perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture 237: 167–178. https://doi.org/10.1016/j.aquaculture.2004.03.018.
93 Jentoft, Sissel, Are H. Aastveit, Peter A. Torjesen, and Øivind Andersen. 2005. Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 141: 353–358. https://doi.org/10.1016/j.cbpb.2005.06.006.
94 Milla, Sylvain, Cédric Mathieu, Neil Wang, Sophie Lambert, Stéphanie Nadzialek, Sophie Massart, Emilie Henrotte, et al. 2010. Spleen immune status is affected after acute handling stress but not regulated by cortisol in Eurasian perch, Perca fluviatilis. Fish & Shellfish Immunology 28: 931–941. https://doi.org/10.1016/j.fsi.2010.02.012.
95 Douxfils, J., S. Lambert, C. Mathieu, S. Milla, S. N. M. Mandiki, E. Henrotte, N. Wang, et al. 2014. Influence of domestication process on immune response to repeated emersion stressors in Eurasian perch (Perca fluviatilis, L.). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 173: 52–60. https://doi.org/10.1016/j.cbpa.2014.03.012.
96 Alanärä, A., and Å. Strand. 2015. The Energy Requirements of Percid Fish in Culture. In Biology and Culture of Percid Fishes: Principles and Practices, ed. P. Kestemont, K. Dabrowski, and Robert C. Summerfelt, 353–369. Dordrecht: Springer Netherlands.
97 Brüning, Anika, Franz Hölker, Steffen Franke, Torsten Preuer, and Werner Kloas. 2015. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Science of The Total Environment 511: 516–522. https://doi.org/10.1016/j.scitotenv.2014.12.094.
98 Strand, Å., C. Magnhagen, and A. Alanärä. 2007. Effects of repeated disturbances on feed intake, growth rates and energy expenditures of juvenile perch, Perca fluviatilis. Aquaculture 265: 163–168. https://doi.org/10.1016/j.aquaculture.2007.01.030.
99 Douxfils, Jessica, S. N. M Mandiki, C. Mathieu, S. Milla, and M. Saroglia. 2015. Domestication and Responses to Stress. In Biology and Culture of Percid Fishes: Principles and Practices, ed. P. Kestemont, K. Dabrowski, and Robert C. Summerfelt, 743–761. Dordrecht: Springer Netherlands.
100 Alix, M., D. Zarski, D. Chardard, P. Fontaine, and B. Schaerlinger. 2017. Deformities in newly hatched embryos of Eurasian perch populations originating from two different rearing systems. Journal of Zoology 302: 126–137. https://doi.org/10.1111/jzo.12447.
101 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
102 Teletchea, Fabrice. 2015. Domestication of Marine Fish Species: Update and Perspectives. Journal of Marine Science and Engineering 3: 1227–1243. https://doi.org/10.3390/jmse3041227.
103 FAO. 2017. FAO Fisheries & Aquaculture - Species Fact Sheets - Perca fluviatilis (Linnaeus, 1758). World Wide Web electronic publication. www.fao.org.
104 Geay, Florian, and Patrick Kestemont. 2015. Feeding and Nutrition of Percid Fishes During Ongrowing Stages. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 587–625. Dordrecht: Springer Netherlands.
105 Tilami, Sarvenaz Khalili, Jan Turek, Daniel Červený, Pavel Lepič, Pavel Kozák, Viktoriia Burkina, Sidika Sakalli, Aleš Tomčala, Sabine Sampels, and Jan Mráz. 2020. Insect Meal as a Partial Replacement for Fish Meal in a Formulated Diet for Perch Perca fluviatilis. Turkish Journal of Fisheries and Aquatic Sciences 20: 867–878. https://doi.org/10.4194/1303-2712-v20_12_03.
106 Tran, Hung Quang, Elena Wernicke von Siebenthal, Jean-Baptiste Luce, Tram Thi Nguyen, Aleš Tomčala, Vlastimil Stejskal, and Thomas Janssens. 2024. Complementarity of insect meal and poultry by-product meal as replacement for fishmeal can sustain the production performance of European perch (Perca fluviatilis), reduce economic fish-in fish-out ratio and food-feed competition, and influence the environmental indices. Aquaculture 579: 740166. https://doi.org/10.1016/j.aquaculture.2023.740166.
107 Stadtlander, T., F. Tschudi, A. Seitz, M. Sigrist, D. Refardt, and F. Leiber. 2023. Partial Replacement of Fishmeal with Duckweed (Spirodela polyrhiza) in Feed for Two Carnivorous Fish Species, Eurasian Perch (Perca fluviatilis) and Rainbow Trout (Oncorhynchus mykiss). Aquaculture Research 2023. https://doi.org/10.1155/2023/6680943.
108 Stejskal, Vlastimil, Hung Quang Tran, Marketa Prokesova, Tatyana Gebauer, Pham Thai Giang, Francesco Gai, and Laura Gasco. 2020. Partially Defatted Hermetia illucens Larva Meal in Diet of Eurasian Perch (Perca fluviatilis) Juveniles. Animals 10: 1876. https://doi.org/10.3390/ani10101876.
109 Tran, Hung Quang, Hien Van Doan, and Vlastimil Stejskal. 2021. Does dietary Tenebrio molitor affect swimming capacity, energy use, and physiological responses of European perch Perca fluviatilis? Aquaculture 539: 736610. https://doi.org/10.1016/j.aquaculture.2021.736610.
110 Tran, Hung Quang, Markéta Prokešová, Mahyar Zare, Jan Matoušek, Ilario Ferrocino, Laura Gasco, and Vlastimil Stejskal. 2022. Production performance, nutrient digestibility, serum biochemistry, fillet composition, intestinal microbiota and environmental impacts of European perch (Perca fluviatilis) fed defatted mealworm (Tenebrio molitor). Aquaculture 547: 737499. https://doi.org/10.1016/j.aquaculture.2021.737499.