Distribution
Information
Version: B | 1.1 (2021-07-17)
Please note: This part of the profile is currently being revised.
1 Remarks
1.1 General remarks
Escapees and consequences: negative or at most unpredictable for the local ecosystem1.2 Other remarks
No data found yet.2 Ethograms
In the farm or lab: on feeding,
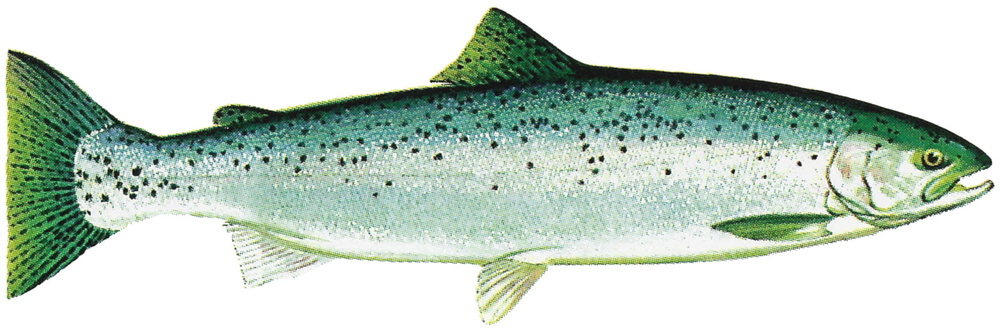
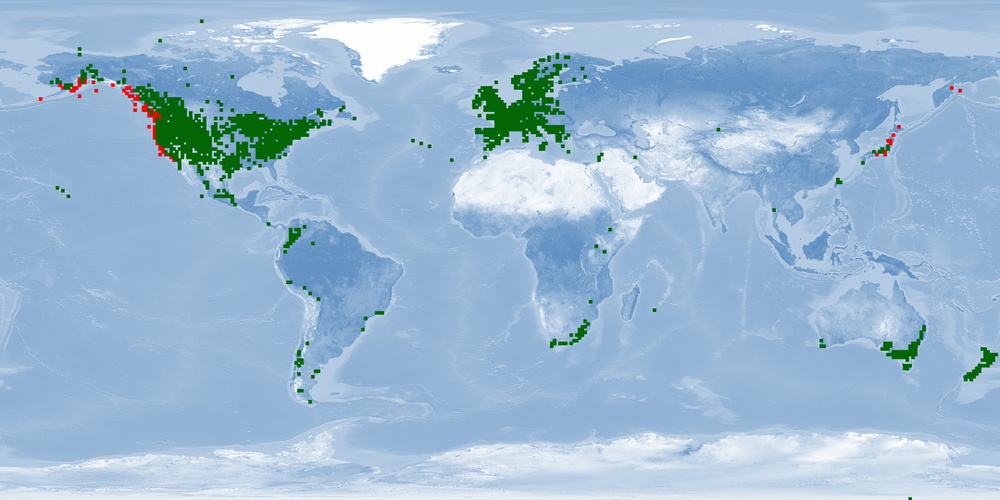
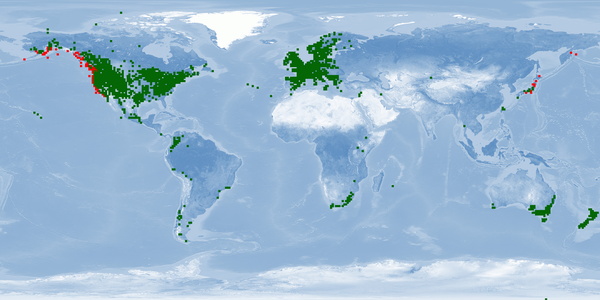
3 Distribution


Natural distribution: North America
Introduced: inland waters worldwide
4 Natural co-existence
5 Substrate and/or shelter
5.1 Substrate
Substrate range, substrate preference: opportunistic – reported from lakes with plants as well as creeks with rocky bottomsSubstrate and stress: inverse effect (further research needed)
Substrate and growth: mixed effects (further research needed)
5.2 Shelter or cover
Shelter or cover preference: plants, woody debris, or boulders for cover from predators, accepts artificial alternatives6 Food, foraging, hunting, feeding
6.1 Trophic level and general considerations on food needs
Trophic level: 4.1Impacts of feed fishery: contributes to overfishing, challenges animal welfare
6.2 Food items
Food items, food preference: carnivorous6.3 Feeding behaviour
Feeding style, foraging mode: depending on turbidity either bottom grazing, surface foraging, or active pursuitFeeding frequency and stress: direct relation (further research needed)
Food competition and stress: direct effect (further research needed)
Food competition and growth: inverse effect (for subordinate individuals)
Effects on feeding: direct relation with temperature, cessation after spawning (further research needed)
For feeding and...
...swimming speed ➝ D6,
...dominance ➝ D7,
...shyness-boldness continuum ➝ D8,
...exploration-avoidance continuum ➝ D9.
7 Photoperiod
7.1 Daily rhythm
Daily rhythm: diurnal but individual differences, nocturnal in winter7.2 Light intensity
No data found yet.7.3 Light colour
Light colour and growth: highest under red light (further research needed)For light colour and confinement ➝ D11.
8 Water parameters
8.1 Water temperature
Standard temperature range, temperature preference: 0-20.5 °C, 10+ °CTemperature and growth: direct relation (further research needed)
8.2 Oxygen
No data found yet.8.3 Salinity
Salinity tolerance, standard salinity range: fresh water (potamodromous Rainbow trout) or fresh to seawater (anadromous Steelhead trout)8.4 pH
No data found yet.8.5 Turbidity
Standard turbidity range: ultra-oligotrophic to eutrophic, Secchi depth 0.5-15 m (further research needed)8.6 Water hardness
No data found yet.8.7 NO4
No data found yet.8.8 Other
No data found yet.9 Swimming
9.1 Swimming type, swimming mode
Swimming type, swimming mode: sub-carangiform9.2 Swimming speed
Swimming speed: 0.01-4.11 km/h, average 0.3-4.2 body lengths/s (further research needed)Standard velocity range, velocity preference: 3-48 cm/s (further research needed)
9.3 Home range
Home range: large-scale movement or site fidelity9.4 Depth
Depth range, depth preference: 0-10 m, up to 100 m, moves deeper with increasing temperatures (further research needed)9.5 Migration
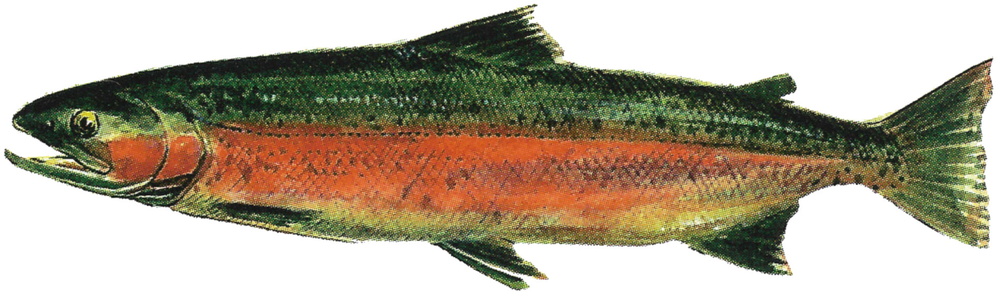
Migration type: anadromous ("Steelhead") or potamodromous form ("Rainbow trout")10 Growth
10.1 Ontogenetic development
Mature egg: 25-36 days from fertilisation until hatching, 0.09-0.13 g (further research needed)Larvae: with Steelhead trout called alevins, hatching to 14-42 days, 17-26 mm fork length, 0.007-0.2 g (further research needed)
Fry: beginning of exogenous feeding (after 2-6 weeks), ca 65 mm, 1.7-2.6 g (further research needed)
Juveniles, sexual maturity: fully developed to beginning of maturity, in Steelhead trout called parr (first summer after hatching) and smolt (from 1-7 years on)
Adults: 4-7 years, 440-590 mm, 1,100-2,100 g, in Steelhead trout called grilse (2-7 years, 40.2-84.6 cm) and kelt
10.2 Sexual conversion
No data found yet.10.3 Sex ratio
Natural male:female ratio: 1:1 (further research needed)10.4 Effects on growth
Growth and other factors: effect of dam and sire (further research needed)For growth and...
...substrate ➝ D23,
...food competition ➝ D24,
...light colour ➝ D25,
...tank colour ➝ D26,
...stocking density ➝ D27,
...acute stress ➝ D11.
10.5 Deformities and malformations
Deformities and malformations: rare: atrophied or partially missing fins, deformed upper or lower jaw (further research needed)11 Reproduction
11.1 Nest building
Nest building: female cuts redd in medium and small gravel, at high water velocity, in 12.7 cm depth (further research needed)11.2 Attraction, courtship, mating
Courtship sequence: male rubs snout over and under female's tail (further research needed)11.3 Spawning
Mating system: polygyny with temporary pair bond (further research needed)Spawning conditions: gravel, October-December until summer, fresh water, high water velocity, 12.7 cm water depth (further research needed)
Male:female ratio resulting in spawning, composition of the broodstock: 1:1.05 (further research needed)
Spawning sequence: female and male release eggs and milt simultaneously (further research needed)
11.4 Fecundity
Female fecundity: 550-1,300 eggs per pit in 6-7 pits per redd (further research needed)11.5 Brood care, breeding
Breeding type: gravel breeder12 Senses
12.1 Vision
Visible spectrum: ultraviolet, blue, green, red (further research needed)Importance of vision: swimming (further research needed)
Tank colour preference: blue or green depending on temperature and acclimation (further research needed)
Tank colour and growth: higher in green and yellow (further research needed)
12.2 Olfaction (and taste, if present)
Importance of olfaction: risk perception (further research needed)12.3 Hearing
Hearing type, hearing spectrum: hearing generalistNoise and stress: no hearing loss, difference in growth, susceptibility to disease from long-term noise (further research needed)
12.4 Touch, mechanical sensing
No data found yet.12.5 Lateral line
Importance of lateral line: adjusting swimming movements (further research needed)12.6 Electrical sensing
No data found yet.12.7 Nociception, pain sensing
Nociception spectrum: polymodal and mechanothermal nociceptors, mechanochemical and mechanical receptors; reacts to low concentrations of acid (further research needed)Pain and treatment: analgesic after painful treatment decreases reactions shown otherwise (further research needed)
12.8 Other
No data found yet.13 Communication
13.1 Visual
No data found yet.13.2 Chemical
Signalling stress: chemical alarm signals positioned in skin (further research needed)13.3 Acoustic
No data found yet.13.4 Mechanical
No data found yet.13.5 Electrical
No data found yet.13.6 Other
No data found yet.14 Social behaviour
14.1 Spatial organisation
Aggregation type: juveniles in schools or solitary, adults solitary or in small groups (further research needed)Stocking density in the wild: 0.01-1.3 ind/m2 (further research needed)
Stocking density and stress: direct relation from ca 75 kg/m3 on (further research needed)
Stocking density and growth: mixed effects (further research needed)
14.2 Social organisation
Social organisation type: linear hierarchy (when in small groups)Features of dominance: occupy and patrol best feeding sites, larger and more aggressive than subordinates
Features of subordination: hardly move, avoid contact with the dominant (further research needed)
14.3 Exploitation
No data found yet.14.4 Facilitation
No data found yet.14.5 Aggression
Aggression and size-grading: attacks, lateral displays, nipping, biting regardless of size-grading (further research needed)For aggression (or lack thereof) and...
...environmental enrichment ➝ D31,
...feeding ➝ D32,
...territoriality ➝ D5.
14.6 Territoriality
Territoriality and feeding: direct effect (further research needed)For territoriality and stress responsiveness selection lines ➝ D8.
15 Cognitive abilities
15.1 Learning
Operant or instrumental conditioning: may be used for feeding, avoidance behaviour (further research needed)15.2 Memory
Memory: conditioning partially works up to seven days after learning trials (further research needed)15.3 Problem solving, creativity, planning, intelligence
No data found yet.15.4 Other
Playing: kelt dart at coins thrown in water, spit them out16 Personality, coping styles
Exploration-avoidance continuum: relationship with feed intake, pain decreases neophobia, stress from novel objects induces hypoalgesia (further research needed)
Aggressiveness continuum: in establishing hierarchy, dominance-subordination, given size-grading
17 Emotion-like states
17.1 Joy
No data found yet.17.2 Relaxation
No data found yet.17.3 Sadness
No data found yet.17.4 Fear
Fear: increase in taking cover, decrease in feeding and swimming, "freezing"18 Self-concept, self-recognition
19 Reactions to husbandry
19.1 Stereotypical and vacuum activities
No data found yet.19.2 Acute stress
Handling: anaesthesia, injection with acid, confinement, transport, chasing, netting is stressful (further research needed)Confinement: stressful if done for 30 min
Live transport: stressful; adding salt to transport water decreases stress (further research needed)
For acute stress and nociception ➝ D9.
19.3 Chronic stress
Effects on welfare: adjust to long-term confinement (further research needed)For chronic stress and...
...substrate ➝ D38,
...feeding frequency ➝ D32,
...food competition ➝ D39,
...noise ➝ D40,
...stocking density ➝ D22.
19.4 Stunning reactions
Stunning rules: fast, effective, safeStunning methods: electrical stunning most effective (further research needed)
Stunning methods and stress: electrical stunning least stressful, air asphyxia most stressful, asphyxia in ice prolongs duration to loss of visual evoked reponses (further research needed)
Glossary
AGGRESSIVENESS = agonistic reactions towards conspecifics. Tests: mirror image, social interaction/diadic encounters 60.
ALEVINS = larvae until the end of yolk sac absorption, for details ➝ Findings 10.1 Ontogenetic development
ANADROMOUS = migrating from the sea into fresh water to spawn
EXPLORATION-AVOIDANCE = reaction to new situations, e.g. new habitat, new food, novel objects. Referred to as neophobia/neophilia elsewhere. Tests: open field, trappability for first time, novel environment, hole board (time spent with head in holes), novel object 60.
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FOOD CONVERSION RATIO = (food offered / weight gained)
FRY = larvae from external feeding on, for details ➝ Findings 10.1 Ontogenetic development
GENERALIST = Generalists detect a narrow bandwidth of sound frequencies (<50-500 Hz, 1,500 Hz max.). High hearing threshold = cannot detect quieter sounds. Typically no swim bladder or no attachment of the swim bladder to the inner ear. Live in loud environments (rivers) 55 56.
GRILSE = adults returning from sea to home river to spawn, for details ➝ Findings 10.1 Ontogenetic development
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
KELTS = adults surviving spawning, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
MILLIARD = 1,000,000,000 40 41
PARR = juvenile stage in rivers, for details ➝ Findings 10.1 Ontogenetic development
POTAMODROMOUS = migrating within fresh water
SHYNESS-BOLDNESS = reaction to risky (but not new!) situations, e.g. predators or humans. Referred to as docility, tameness, fearfulness elsewhere. Tests: predator presentation, predator stimulus, threat, trappability (latency to enter a trap for first time can be exploration), resistance to handlers (Trapezov stick test), tonic immobility (catatonic-like death-feigning anti predator response) 60.
SMOLTS = juvenile stage migrating to the sea, for details ➝ Findings 10.1 Ontogenetic development
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) 48 or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson)
WILD = setting in the wild
Bibliography
2 Patterson, Kristen, and Paul J. Blanchfield. 2013. Oncorhynchus mykiss escaped from commercial freshwater aquaculture pens in Lake Huron, Canada. Aquaculture Environment Interactions 4: 53–65. https://doi.org/10.3354/aei00073.
3 McMichael, Geoffrey A., Cameron S. Sharpe, and Todd N. Pearsons. 1997. Effects of Residual Hatchery-Reared Steelhead on Growth of Wild Rainbow Trout and Spring Chinook Salmon. Transactions of the American Fisheries Society 126: 230–239. https://doi.org/10.1577/1548-8659(1997)126<0230:EORHRS>2.3.CO;2.
4 McMichael, Geoffrey A., Todd N. Pearsons, and Steven A. Leider. 1999. Behavioral Interactions among Hatchery-Reared Steelhead Smolts and Wild Oncorhynchus mykiss in Natural Streams. North American Journal of Fisheries Management 19: 948–956. https://doi.org/10.1577/1548-8675(1999)019<0948:BIAHRS>2.0.CO;2.
5 Boyer, Matthew C, Clint C Muhlfeld, and Fred W Allendorf. 2008. Rainbow trout (Oncorhynchus mykiss) invasion and the spread of hybridization with native westslope cutthroat trout (Oncorhynchus clarkii lewisi). Canadian Journal of Fisheries and Aquatic Sciences 65: 658–669. https://doi.org/10.1139/f08-001.
6 Shapovalov, Leo, and Alan C. Taft. 1954. The Life Histories of the Steelhead Rainbow Trout (Salmo gairdneri gairdneri) and Silver Salmon (Oncorhynchus kisutch) With Special Reference to Waddell Creek, California, and Recommendations Regarding Their Management. Fish Bulletin 98. State of California Department of Fish and Game.
7 Bradford, Michael J, and Paul S Higgins. 2001. Habitat-, season-, and size-specific variation in diel activity patterns of juvenile chinook salmon (Oncorhynchus tshawytscha) and steelhead trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences 58: 365–374. https://doi.org/10.1139/f00-253.
8 Riehle, Michael D., and J. S. Griffith. 1993. Changes in Habitat Use and Feeding Chronology of Juvenile Rainbow Trout (Oncorhynchus mykiss) in Fall and the Onset of Winter in Silver Creek, Idaho. Canadian Journal of Fisheries and Aquatic Sciences 50: 2119–2128. https://doi.org/10.1139/f93-237.
9 Meyer, K. A., and J. S. Gregory. 2000. Evidence of concealment behavior by adult rainbow trout and brook trout in winter. Ecology of Freshwater Fish 9: 138–144. https://doi.org/10.1111/j.1600-0633.2000.eff090302.x.
10 Light, Jeffrey T, Cohn K Harris, and Robert L Burgner. 1989. Ocean distribution and migration of steelhead (Oncorhynchus mykiss, formerly Salmo gairdneri). Document submitted to the International North Pacific Fisheries Commission. FRI-UW-8912. Seattle: FisheriesResearch Institute, University of Washington.
11 James, G. D., and J. R. M. Kelso. 1995. Movements and habitat preference of adult rainbow trout (Oncorhynchus mykiss) in a New Zealand montane lake. New Zealand Journal of Marine and Freshwater Research 29: 493–503. https://doi.org/10.1080/00288330.1995.9516682.
12 Del Real, S. Casey, Michelle Workman, and Joseph Merz. 2012. Migration characteristics of hatchery and natural-origin Oncorhynchus mykiss from the lower Mokelumne River, California. Environmental Biology of Fishes 94: 363–375. https://doi.org/10.1007/s10641-011-9967-z.
13 Hayes, Sean A., Morgan H. Bond, Chad V. Hanson, Andrew W. Jones, Arnold J. Ammann, Jeffrey A. Harding, Alison L. Collins, Jeffrey Perez, and R. Bruce MacFarlane. 2011. Down, up, down and “smolting” twice? Seasonal movement patterns by juvenile steelhead (Oncorhynchus mykiss) in a coastal watershed with a bar closing estuary. Canadian Journal of Fisheries and Aquatic Sciences 68: 1341–1350. https://doi.org/10.1139/f2011-062.
14 Berejikian, Barry A, E Paul Tezak, Thomas A Flagg, Anita L LaRae, Eric Kummerow, and Conrad VW Mahnken. 2000. Social dominance, growth, and habitat use of age-0 steelhead (Oncorhynchus mykiss) grown in enriched and conventional hatchery rearing environments. Canadian Journal of Fisheries and Aquatic Sciences 57: 628–636. https://doi.org/10.1139/f99-288.
15 Bégout Anras, Marie-Laure, and Jean Paul Lagardère. 2004. Measuring cultured fish swimming behaviour: first results on rainbow trout using acoustic telemetry in tanks. Aquaculture 240: 175–186. https://doi.org/10.1016/j.aquaculture.2004.02.019.
16 Gregory, T Ryan, and Chris M Wood. 1999. Interactions between individual feeding behaviour, growth, and swimming performance in juvenile rainbow trout (Oncorhynchus mykiss) fed different rations. Canadian Journal of Fisheries and Aquatic Sciences 56: 479–486. https://doi.org/10.1139/f98-186.
17 Liao, James C. 2006. The role of the lateral line and vision on body kinematics and hydrodynamic preference of rainbow trout in turbulent flow. Journal of Experimental Biology 209: 4077–4090. https://doi.org/10.1242/jeb.02487.
18 Boglione, Clara, Domitilla Pulcini, Michele Scardi, Elisa Palamara, Tommaso Russo, and Stefano Cataudella. 2014. Skeletal Anomaly Monitoring in Rainbow Trout (Oncorhynchus mykiss , Walbaum 1792) Reared under Different Conditions. PLOS ONE 9: e96983. https://doi.org/10.1371/journal.pone.0096983.
19 Øverli, Øyvind, Charmaine A. Harris, and Svante Winberg. 1999. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain, Behavior and Evolution 54: 263–275. https://doi.org/10.1159/000006627.
20 Toobaie, Asra, and James W. A. Grant. 2013. Effect of food abundance on aggressiveness and territory size of juvenile rainbow trout, Oncorhynchus mykiss. Animal Behaviour 85: 241–246. https://doi.org/10.1016/j.anbehav.2012.10.032.
21 Basic, D., S. Winberg, J. Schjolden, Å. Krogdahl, and E. Höglund. 2012. Context-dependent responses to novelty in Rainbow trout (Oncorhynchus mykiss), selected for high and low post-stress cortisol responsiveness. Physiology & Behavior 105: 1175–1181. https://doi.org/10.1016/j.physbeh.2011.12.021.
22 Sneddon, Lynne U, Victoria A Braithwaite, and Michael J Gentle. 2003. Novel object test: examining nociception and fear in the rainbow trout. The Journal of Pain 4: 431–440. https://doi.org/10.1067/S1526-5900(03)00717-X.
23 Yue, S, R. D Moccia, and I. J. H Duncan. 2004. Investigating fear in domestic rainbow trout, Oncorhynchus mykiss, using an avoidance learning task. Applied Animal Behaviour Science 87: 343–354. https://doi.org/10.1016/j.applanim.2004.01.004.
24 Sneddon, L. U. 2003. The bold and the shy: individual differences in rainbow trout. Journal of Fish Biology 62: 971–975. https://doi.org/10.1046/j.1095-8649.2003.00084.x.
25 Brown, Grant E., and R. Jan F. Smith. 1997. Conspecific skin extracts elicit antipredator responses in juvenile rainbow trout (Oncorhynchus mykiss). Canadian Journal of Zoology 75: 1916–1922. https://doi.org/10.1139/z97-821.
26 Reilly, Siobhan C., John P. Quinn, Andrew R. Cossins, and Lynne U. Sneddon. 2008. Behavioural analysis of a nociceptive event in fish: Comparisons between three species demonstrate specific responses. Applied Animal Behaviour Science 114: 248–259. https://doi.org/10.1016/j.applanim.2008.01.016.
27 Reviewed distribution maps for Rainbow trout (Oncorhynchus mykiss). 2016. Aquamaps.
28 Alagona, Peter S., Scott D. Cooper, Mark Capelli, Matthew Stoecker, and Peggy H. Beedle. 2012. A History of Steelhead and Rainbow Trout (Oncorhynchus mykiss) in the Santa Ynez River Watershed, Santa Barbara County, California. Bulletin, Southern California Academy of Sciences 111: 163–222. https://doi.org/10.3160/0038-3872-111.3.163.
29 Rowe, D. K. 1984. Factors affecting the foods and feeding patterns of lake‐dwelling rainbow trout (Salmo gairdnerii) in the North Island of New Zealand. New Zealand Journal of Marine and Freshwater Research 18: 129–141. https://doi.org/10.1080/00288330.1984.9516036.
30 Kusabs, Ian A., and Stephen Swales. 1991. Diet and food resource partitioning in koaro, Galaxias brevipinnis (Günther), and juvenile rainbow trout, Oncorhynchus mykiss (Richardson), in two Taupo streams, New Zealand. New Zealand Journal of Marine and Freshwater Research 25: 317–325. https://doi.org/10.1080/00288330.1991.9516485.
31 NOT FOUND
32 Arndt, Ronney E., M. Douglas Routledge, Eric J. Wagner, and Roger F. Mellenthin. 2002. The use of AquaMats® to enhance growth and improve fin condition among raceway cultured rainbow trout Oncorhynchus mykiss (Walbaum). Aquaculture Research 33: 359–367. https://doi.org/10.1046/j.1365-2109.2002.00670.x.
33 Shapovalov, Leo. 1937. Experiments in hatching steelhead eggs in gravel. California Fish and Game 23: 208–214.
34 Becket, Kristen H, and Michael Barnes. 2015. Rearing with overhead cover influences rainbow trout behavior. Proceedings of the South Dakota Academy of Science 94: 187–193.
35 Froese, R., and D. Pauly. 2014. FishBase. World Wide Web electronic publication. www.fishbase.org.
36 FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organization of the United Nations.
37 Watson, R., Jackie Alder, and Daniel Pauly. 2006. Fisheries for forage fish, 1950 to the present. In On the Multiple Uses of Forage Fish: from Ecosystems to Markets, ed. Jackie Alder and Daniel Pauly, 14:1–20. Fisheries Centre Research Reports 3. Vancouver, Canada: Fisheries Centre, University of British Columbia.
38 Mood, A. 2012. Average annual fish capture for species mostly used for fishmeal (2005-2009). fishcount.org.uk.
39 Mood, A., and P. Brooke. 2012. Estimating the Number of Farmed Fish Killed in Global Aquaculture Each Year.
40 Kopf, Von Kristin. 2012. Milliarden vs. Billionen: Große Zahlen. Sprachlog.
41 Weisstein, Eric W. 2018. Milliard. Text. MathWorld - a Wolfram Web resource. http://mathworld.wolfram.com/Milliard.html. Accessed February 2.
42 Klíma, Ondřej, Lukáš Kohút, Jan Mareš, and Radovan Kopp. 2018. The Effect of Feeding Frequency on the Fin Condition in Rainbow Trout (Oncorhynchus mykiss). Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 66: 669–675. https://doi.org/10.11118/actaun201866030669.
43 Karakatsouli, Nafsika, Sofronios E. Papoutsoglou, Georgios Panopoulos, Eustratios S. Papoutsoglou, Stella Chadio, and Dimitris Kalogiannis. 2008. Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacultural Engineering 38: 36–42. https://doi.org/10.1016/j.aquaeng.2007.10.006.
44 Hunt, Darcie Elizabeth. 2015. The effect of visual capacity and swimming ability of fish on the performance of light-based bycatch reduction devices in prawn trawls. Doctoral dissertation, University of Tasmania.
45 Ruggerone, G., and T. P. Quinn. 1989. Unpublished data.
46 Hawkins, Denise K, and Chris J Foote. 1998. Early survival and development of coastal cutthroat trout (Oncorhynchus clarki clarki), steelhead (Oncorhynchus mykiss), and reciprocal hybrids. Canadian Journal of Fisheries and Aquatic Sciences 55: 2097–2104. https://doi.org/10.1139/f98-099.
47 Wysocki, Lidia Eva, John W. Davidson, Michael E. Smith, Adam S. Frankel, William T. Ellison, Patricia M. Mazik, Arthur N. Popper, and Julie Bebak. 2007. Effects of aquaculture production noise on hearing, growth, and disease resistance of rainbow trout Oncorhynchus mykiss. Aquaculture 272: 687–697. https://doi.org/10.1016/j.aquaculture.2007.07.225.
48 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
49 NOT FOUND
50 Kestin, S. C., S. B. Wotton, and N. G. Gregory. 1991. Effect of slaughter by removal from water on visual evoked activity in the brain and reflex movement of rainbow trout (Oncorhynchus mykiss). The Veterinary Record 128: 443–446.
51 Luchiari, A. C., and J. Pirhonen. 2008. Effects of ambient colour on colour preference and growth of juvenile rainbow trout Oncorhynchus mykiss (Walbaum). Journal of Fish Biology 72: 1504–1514. https://doi.org/10.1111/j.1095-8649.2008.01824.x.
52 Bosakowski, Thomas, and Eric J. Wagner. 1994. Assessment of Fin Erosion by Comparison of Relative Fin Length in Hatchery and Wild Trout in Utah. Canadian Journal of Fisheries and Aquatic Sciences 51: 636–641. https://doi.org/10.1139/f94-064.
53 Needham, P. R., and Alan C. Taft. 1934. Observations on the spawning of steelhead trout. Transactions of the American Fisheries Society 64: 332–338.
54 Hawryshyn, Craig W., and Ferenc I. Hárosi. 1994. Spectral characteristics of visual pigments in rainbow trout (Oncorhynchus mykiss). Vision Research 34. The Biology of Ultraviolet Reception: 1385–1392. https://doi.org/10.1016/0042-6989(94)90137-6.
55 Brown, Culum. 2015. Fish intelligence, sentience and ethics. Animal Cognition 18: 1–17. https://doi.org/10.1007/s10071-014-0761-0.
56 Amundsen, Lasse, and Martin Landro. 2011. Marine seismic sources part VIII: Fish hear a great deal. Recent Advances in Technology 8: 1–5.
57 Ashley, Paul J., Lynne U. Sneddon, and Catherine R. McCrohan. 2007. Nociception in fish: stimulus–response properties of receptors on the head of trout Oncorhynchus mykiss. Brain Research 1166: 47–54. https://doi.org/10.1016/j.brainres.2007.07.011.
58 North, B. P., J. F. Turnbull, T. Ellis, M. J. Porter, H. Migaud, J. Bron, and N. R. Bromage. 2006. The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture 255: 466–479. https://doi.org/10.1016/j.aquaculture.2006.01.004.
59 Pickering, A. D., T. G. Pottinger, J. P. Sumpter, J. F. Carragher, and P. Y. Le Bail. 1991. Effects of acute and chronic stress on the levels of circulating growth hormone in the rainbow trout, Oncorhynchus mykiss. General and Comparative Endocrinology 83: 86–93. https://doi.org/10.1016/0016-6480(91)90108-I.
60 Réale, Denis, Simon M. Reader, Daniel Sol, Peter T. McDougall, and Niels J. Dingemanse. 2007. Integrating animal temperament within ecology and evolution. Biological Reviews 82: 291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x.
61 Tacchi, Luca, Liam Lowrey, Rami Musharrafieh, Kyle Crossey, Erin T. Larragoite, and Irene Salinas. 2015. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss). Aquaculture 435: 120–127. https://doi.org/10.1016/j.aquaculture.2014.09.027.
62 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
63 Lines, J. A., D. H. Robb, S. C. Kestin, S. C. Crook, and T. Benson. 2003. Electric stunning: a humane slaughter method for trout. Aquacultural Engineering 28: 141–154. https://doi.org/10.1016/S0144-8609(03)00021-9.
64 Robb, D. H. F, M O’ Callaghan, J. A Lines, and S. C Kestin. 2002. Electrical stunning of rainbow trout (Oncorhynchus mykiss): factors that affect stun duration. Aquaculture 205: 359–371. https://doi.org/10.1016/S0044-8486(01)00677-9.
65 Concollato, Anna, Rolf Erik Olsen, Sheyla Cristina Vargas, Antonio Bonelli, Marco Cullere, and Giuliana Parisi. 2016. Effects of stunning/slaughtering methods in rainbow trout (Oncorhynchus mykiss) from death until rigor mortis resolution. Aquaculture 464: 74–79. https://doi.org/10.1016/j.aquaculture.2016.06.009.
❮
❯






«